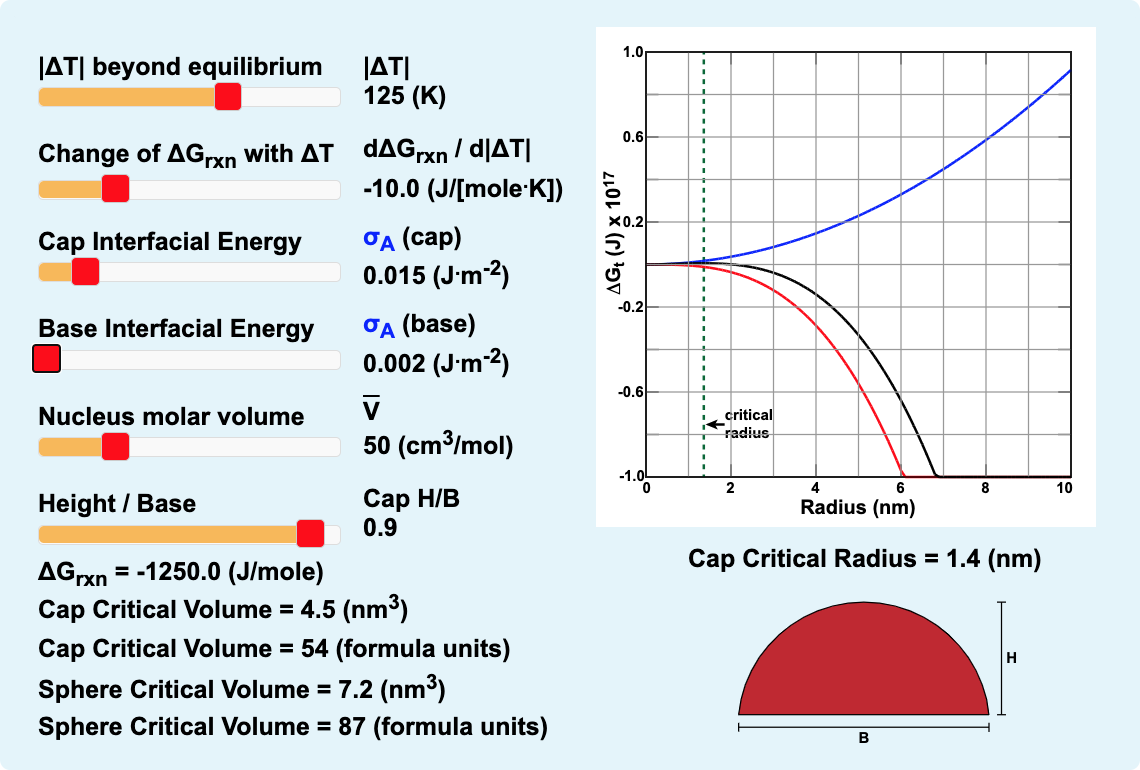
Figure 2.07. Spherical Cap Nucleation. Click on the image to see a larger version with sliders to change the calculation parameters along with a more detailed explanation.
The model of Figure 2.07 is similar to the model of Figure 2.06 with additional sliders to set the interfacial energy of the base of a spherical cap and to change the height (H) to base (B) ratio (H/B) for the nucleus. Click on Figure 2.07 to open a larger version and move the |ΔT| and slider to 125°C. The other parameters are preset to match those from the question on the previous page concerning the kyanite to sillimanite reaction . Note that both the hemisphere cap nucleus and the spherical nuclues have a 1 nm critical radius, but only half the number of sillimanite formula units as a spherical nucleus. Now move the slider to add interfacial energy for the base of the spherical cap. And/or move the slider to change the shape (H/B) of the spherical cap.
If the spherical cap shape factor (H/A) is reduced, will the critical volume of the spherical cap increase or decrease? Please make a choice from the following list and press "Enter":
Your answer is incorrect. Refresh Figure 2.07, move the |ΔT| and slider to 125°C, use the slider to change the geometric factor (H/A) and watch the numbers for the "Cap Critical Volume." Please try again.
If the spherical cap shape factor (H/A) is reduced, will the critical volume of the spherical cap increase or decrease? Please make a choice from the following list and press "Enter": If the spherical cap shape factor (H/A) is reduced, will the critical volume of the spherical cap increase or decrease? Please make a choice from the following list and press "Enter":
No. You still have not given the correct answer. IThe smaller the (H/A) value, the larger the critical volume will be for a spherical cap nucleus.
Yes! The smaller the (H/A) value, the larger the critical volume will be for a spherical cap nucleus.
When the interfacial energy per unit area is raised for the base of the spherical cap, the critical volume of a spherical cap nucleus increases, as does the number of formula units of sillimanite in that nucleus. If the number of formula units of Si2O5 in the critical size spherical cap nucleus is to be less than the number of formula units of Si2O5 in the critical size spherical nucleus, how much smaller must the interfacial energy (J/m2) be for the base than the interfacial energy (J/m2) for the cap,? Please make a choice from the following list and press "Enter":
Your answer is incorrect. Refresh Figure 2.07, move the |ΔT| and slider to 125°C, use the slider to change the base interfacial energy σA (base), and watch the formula unit numbers for the "Cap Critical Volume" and the "Sphere Critical Volume". Please try again.
If the number of formula units of Si2O5 in the critical size spherical cap nucleus is to be less than the number of formula units of Si2O5 in the critical size spherical nucleus, how much smaller must the interfacial energy (J/m2) be for the base than the interfacial energy (J/m2) for the cap,? Please make a choice from the following list and press "Enter":
No. You still have not given the correct answer. If the interfacial energy of the base (J/m2) of a spherical cap nucleus is less than half the interfacial energy of the cap (J/m2), then the number of formula units of the product phase in the critical spherical cap nucleus is less than the number of formula units of the product phase in the critical spherical nucleus.
Yes! If the interfacial energy of the base (J/m2) of a spherical cap nucleus is less than half the interfacial energy of the cap (J/m2), then the number of formula units of the product phase in the critical spherical cap nucleus is less than the number of formula units of the product phase in the critical spherical nucleus.
Coming...