A major control on the rates of most petrologic processes is temperature. Higher temperatures mean higher reaction rates, higher crystal growth rates, higher diffusion rates, etc. Why? What do higher temperatures do to rocks and minerals that speed things up? The temperature of an object is a meaure of the average kinetic energy of the movements or vibrations of atoms in that object. Temperature increases lead to increases in the motion of atoms and, therefore, increases in the internal energy of minerals and other materials.
Evidence for the motion of atoms in hot rocks includes the energy they emit as infrared radiation and, if hot enough, visible light. Very hot rocks and lava visibly glow during volcanic eruptions (see Figure 10 or watch some volcano videos).

Figure 12. Crested Pool Infrared Overlay. A false color infrared image is superimposed on a photograph of a hotspring pool. Click on the image for a larger, interactive version with more information.
Vibrational motions of atoms in minerals, even at low temperatures, seem at first to be at odds with those static, ball and stick crystal structure models often used in mineralogy courses. But the infrared and visible radiation of minerals at higher temperatures requires dynamic, rapidly moving atoms. Although we don't have a way to photograph those motions, it is possible to calculate what they might look like
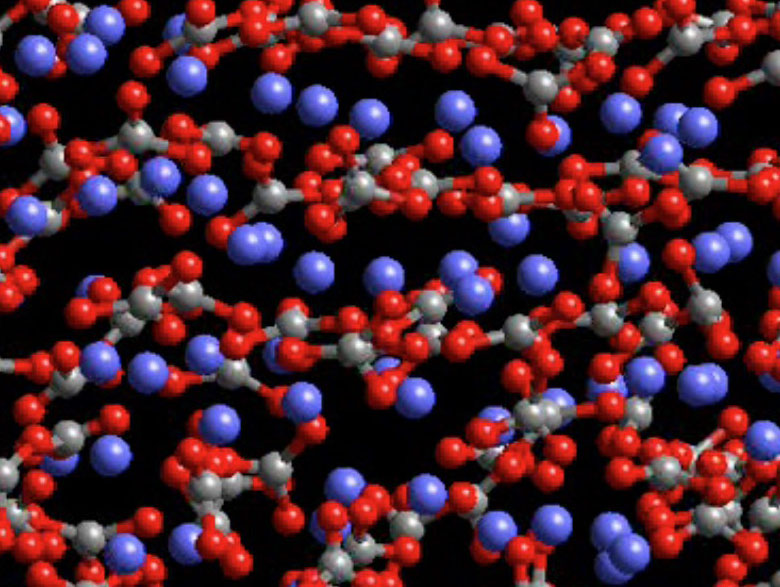
Figure 13. Calcite at 1200 K. Click on the image for a larger, animated, interactive version with more information.
Although minerals are not gases, some aspects of the kinetic theory of gases can help us understand the effect of temperature on the rates of petrologic processes.
