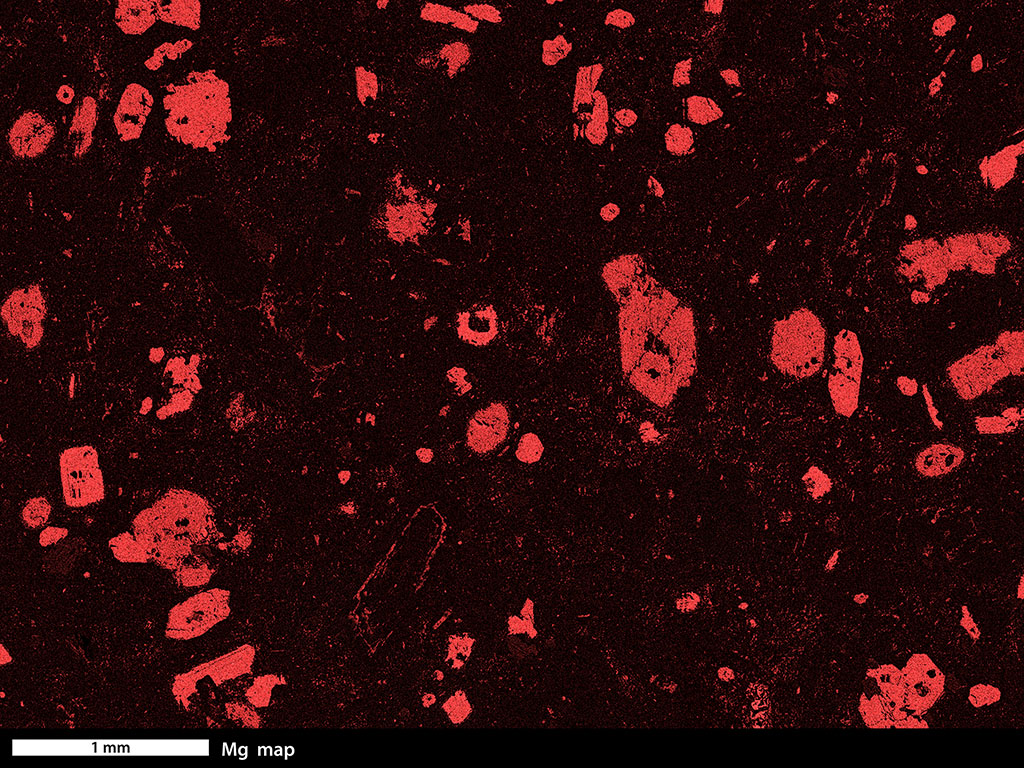
Evidence for chemical diffusion can be found in almost every igneous and metamorphic rock. For example, the images at the top of this page both show rocks with larger crystals in a finer-grained matrix. In each case, the larger crystals have distinctly different chemical compositions than the matrix that surrounds them. To grow those crystals, some atoms must have moved toward the site of the growing crystal and and other atoms must have moved away. Click on Figure 17 and select the Fe map button. You will see that the garnet porphyroblasts in this metabasalt are rich in iron, relative to their matrix. Click on the Mn button and you will see that the cores of the garnet porphyroblasts are especially rich in manganese. Figure 18 is a Mg map of an andesite. Click on Figure 18 and try the Mg and Ca buttons to see ways that the different elements are distributed.
As discussed already, atoms in minerals are always in motion, vibrating in place with a statistical distribution of energies that increases with temperature. Atoms with higher energies might break their bonds and move to another location. What would cause them to move one direction instead of another? One way to understand this is to examine random motions of the atoms in a gas.
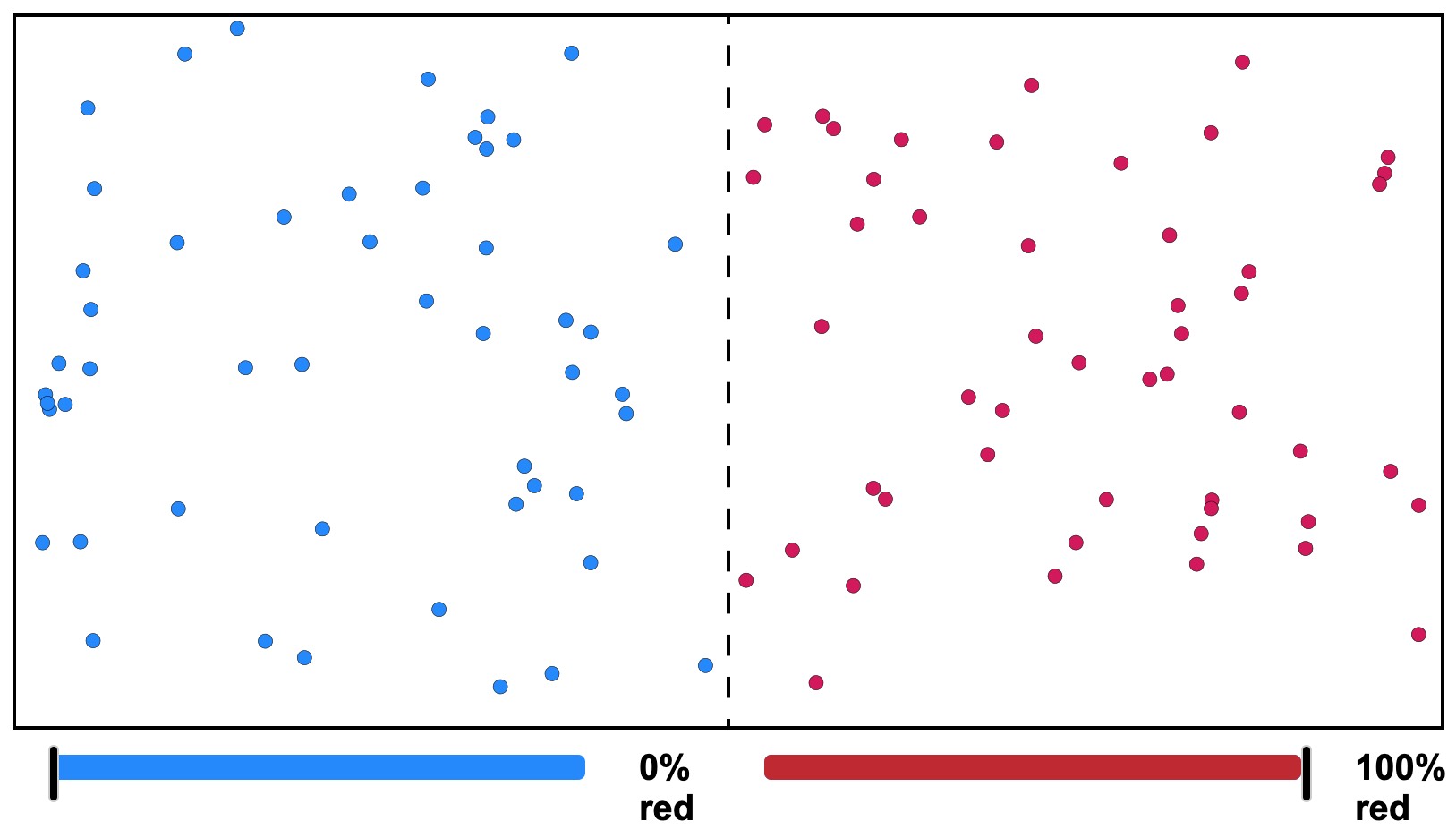
Figure 19. Gas Diffusion Model. A divided chamber with colored gas atoms. What will happen if the partition is removed? Click on the image for a larger, interactive version where you can observe what happens.
Diffusion is different than flow. Atoms move together in a flowing pore fluid or a flowing magma, but they do not change their proportions because of the flow. When atoms move randomly where there or differences or gradients in chemical concentrations, chemical proportions are changed and the process is diffusion.