Olivine is a common mineral with a chemical composition that ranges from pure Mg-olivine (forsterite) to pure Fe-olivine (fayalite). Other elements, notably Mn, can be found in olivine, but olivine compositions are largely a two-component mixture of forsterite and fayalite. A phase diagram based on the melting of olivine looks a lot like the water-ethanol boiling diagram. Figure 5.03 shows the olivine melting diagram determined by Bowen and Schairer (1935).
Figure 5.03. Olivine melting diagram. Phase relations for olivine melting as a function of olivine compostion at 1 bar pressure. Click on the diagram to see a larger, interactive version.
If you follow the melting of olivine using the "Show Phase %" button on the olivine melting diagram, you will see that the liquid that forms is richer in iron than the crystal that is melting. This means that the olivine crystal that is melting must change its chemical composition to become more magnesium rich. This is more difficult to do for solid olivine than it is for a water-ethanol solution to change its composition during boiling. The olivine might change by regrowing as a new, more forsteritic olivine. Or it might change through diffusional exchange of Mg and Fe. Time and high temperatures both facilitate the changes needed to maintain crystal-liquid equilibrium.
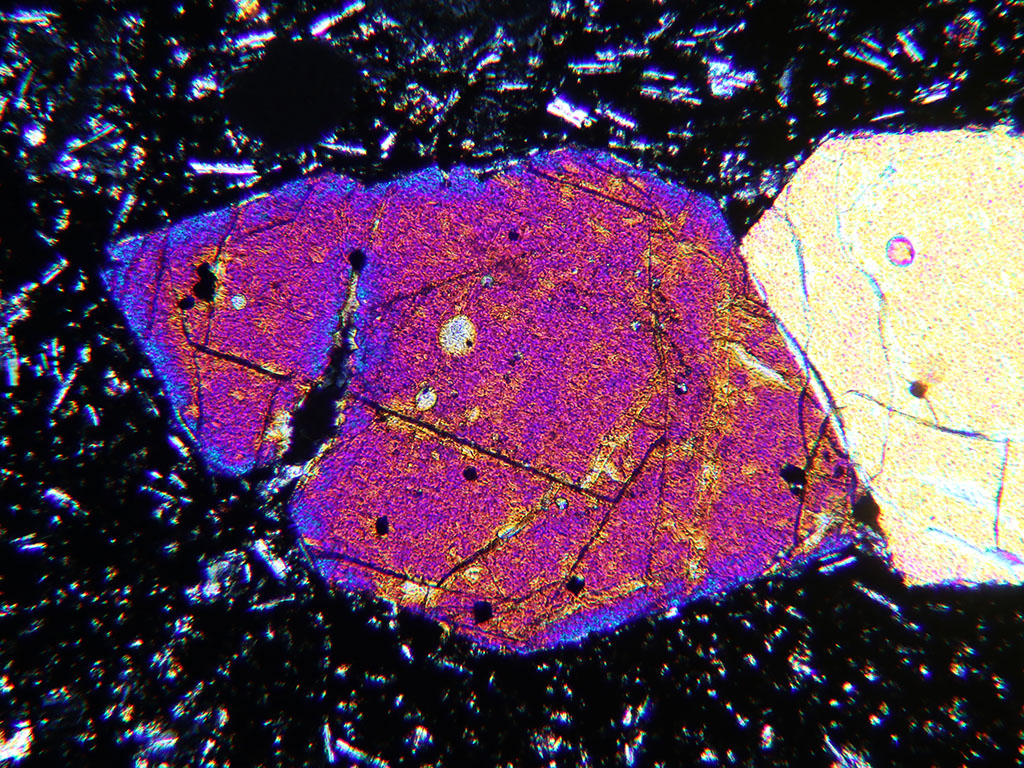
Figure 5.04. Fe-rich rim on olivine. Crossed polarized light image of olivine phenocryst in basalt from Mauna Loa, Hawaii. The second-order blue retardation of the rim on the first-order red olivine crystal is due to the increased iron content of the rim. The crystal is 0.8 mm long.
You may use Figure 5.03 and its "Coordinates" and "Show Phase %" buttons to help you find the answer this question. Press "Enter" after you type in the number.
Yes. The liquidus temperature for an Mg2SiO4-Fe2SiO4 liquid that is 40 wt% Fe2SiO4, is about 1728°C.
No. Use the "Coordinates" button on Figure 5.03 to find the temperature where the 40 wt% Fe2SiO4 vertical line crosses the liquidus and try again.
Yes. The liquidus temperature for an Mg2SiO4-Fe2SiO4 liquid that is 40 wt% Fe2SiO4, is about 1728°C.
No. The liquidus temperature for an Mg2SiO4-Fe2SiO4 liquid that is 40 wt% Fe2SiO4, is about 1728°C. You should be able to answer this correctly. Reload the page and use the "Coordinates" and "Show Phase %" buttons on Figure 5.03 to make sure you can find the liquidus temperature.
Yes. At equilibrium, the chemical composition of the first olivine crystals formed by cooling a 40 wt% Fe2SiO4 liquid will be 14 wt% Fe2SiO4.
No. At equilibrium, the chemical composition of the first olivine crystals formed by cooling a 40 wt% Fe2SiO4 liquid will be 14 wt% Fe2SiO4. Use the "Coordinates" and "Show Phase %" buttons on Figure 5.03 to find the solidus composition at 1728°C.