Melting and crystallization of solid solution minerals has many similarities to the boiling of water-alcohol mixtures. Because boiling of alcohol-bearing solutions is easy to do in the laboratory, we will examine an example of boiling to help understand the melting of solid solution minerals like olivine and plagioclase.
Yeast is a fungus that turns sugar into alcohol and CO2 and uses the energy released by this fementation reaction to multiply. Long ago humans learned to use yeast to turn grapes into wine and barley into beer. Too much alcohol is toxic to yeast, so fermentation stops when the alcohol content of the must (wine) or wort (beer) reachs 13-18 volume percent ethanol (ABV), depending on the particular yeast strain.
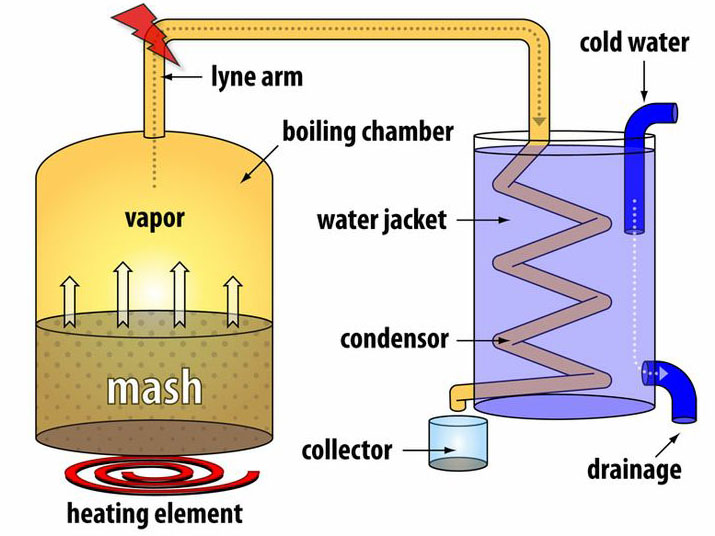
Figure 5.01. Schematic diagram of a simple moonshine still. The mash (water-alcohol-grain mixture) is boiled in the chamber on the left, condensed in the cooling coil, and the resulting liquid drips into the collector pot. Click on the diagram for a larger version with more information.
Equipment that is used to separate and condense vapor from a boiling liquid is called a "still" (from "distill"). Stills are used to change the chemical composition of a solution. For example, a still might be used to make "moonshine," an alcohol-rich beverage, from a liquid less concentrated in alcohol. Figure 5.01, taken from a book about moonshine, shows a model, water-cooled still. Similar stills are used for a variety of chemical processes, including simple purification of water. Partial melting of solid solution minerals along with separation and crystallization of the melt also can lead to a change of chemical composition.