Alkali feldspar is a solid solution mineral ranging in composition from Na-rich (albite) to K-rich (sanidine). The alkali feldspar melting diagram (Figure 5.07) has an azeotropic point. Upon cooling, melts rich in potassium crystallize alkali feldspar (sanidine) that is even more K-rich.
Figure 5.07. Alakli Feldspar Phase Diagram. The liquidus and solidus for the alkali feldspar solid solution meet at an azeotropic (minimum) point. This relationship is typical for minerals that have a solvus at lower temperatures. Click on the diagram to see a larger, interactive version .
When an alkali feldspar of intermediate composition is cooled slowly into the two-felspar region, the single feldspar will unmix (exsolve)and form a perthite. A perthite is an intergrowth of orthoclase and albite (or microcline and albite) formed from a single alkali feldspar crystal during cooling. Examples of perthite in thin section are shown in Figure 5.08 .
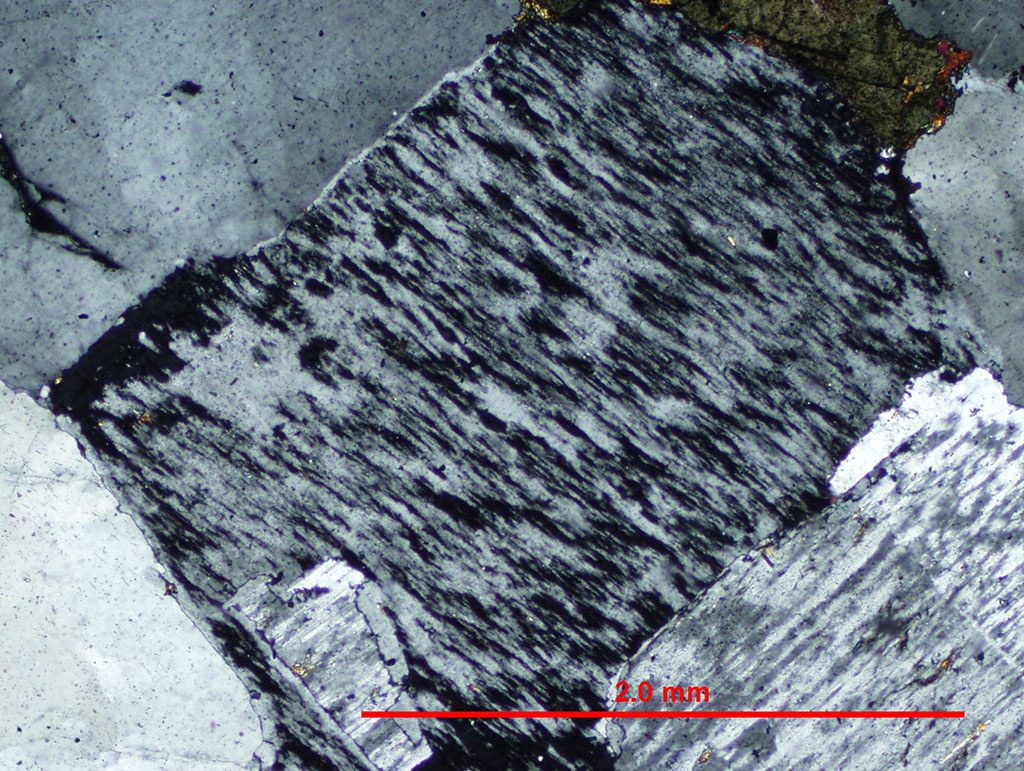
Figure 5.08. Perthite. Microperthite in a thin section of an alkali feldspar granite from Cape Ann, Massachusetts. In the crossed polarized light (xpl) image, the albite portion of the crystal is at extinction showing clearly the complex intergrowth texture.
Water under pressure (PH2O) changes the melting temperature for alkali feldspar. This effect is shown on the large version of Figure 5.07 if you use the pressure slider. PH2O has little effect on the alkali feldspar solvus, so the lowered solidus intercepts the solvus creating a solid solution eutectic.