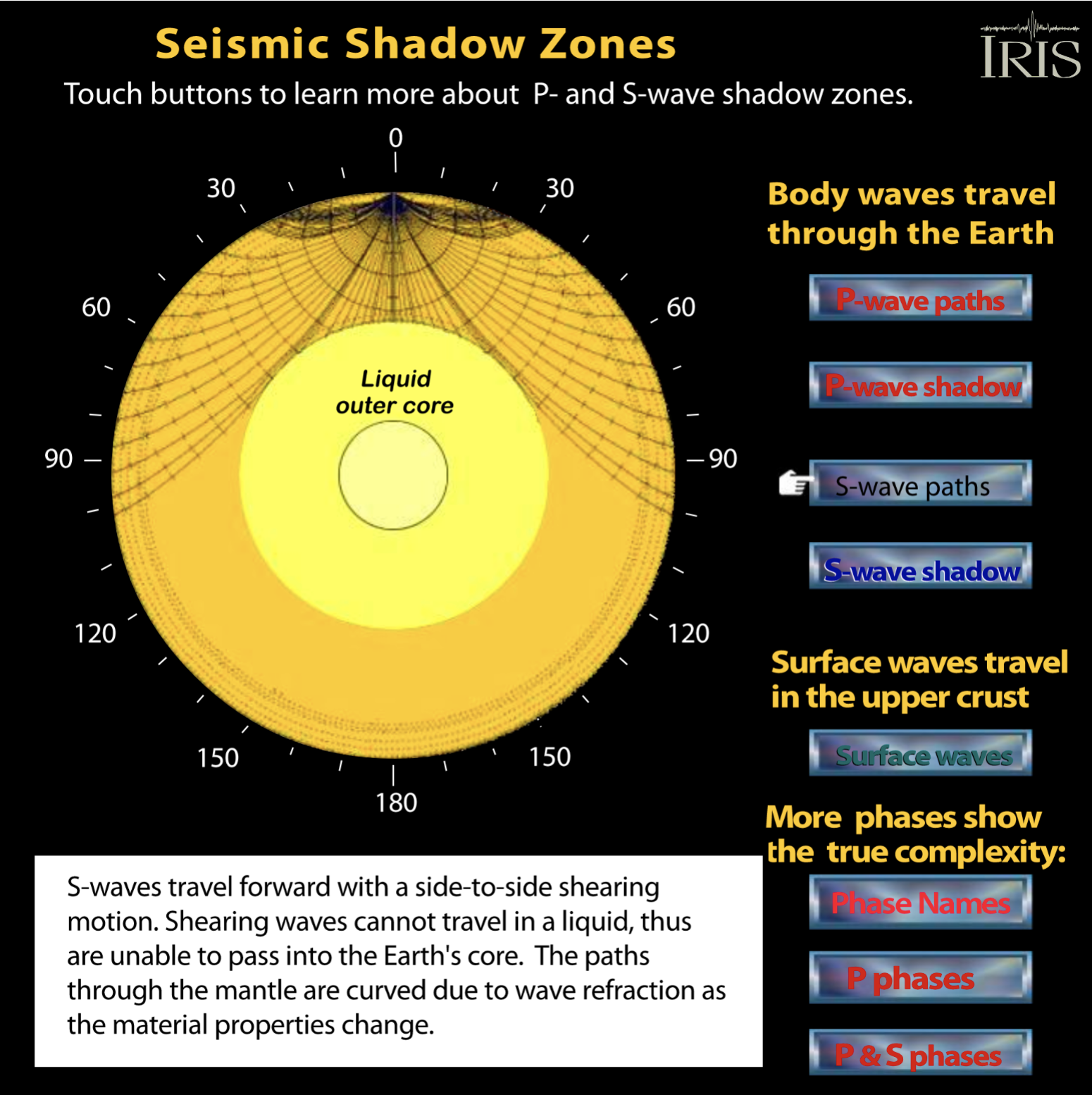
Plate tectonics models of the earth show the mantle convecting, flowing upward beneath divergent plate boundaries and flowing downward at convergent plate boundaries. Some people incorrectly interpret this to mean that the mantle is a liquid. Look, for example, at this image that suggests a molten mantle taken originally from an online article. Although the mantle flows, it consists of solid minerals that flow ductilely. We know this from seismic data. Sound travels through the earth as compressional waves (P-waves) and as shear waves (S-waves). S-waves are not transmitted by liquids. S-waves DO travel through the mantle, so the mantle must be solid. S-waves do not pass through the Earth's core. This means that the core, or at least the outer core, must be liquid. See the good S-wave image in Figure 2.04, modified from the IRIS website).
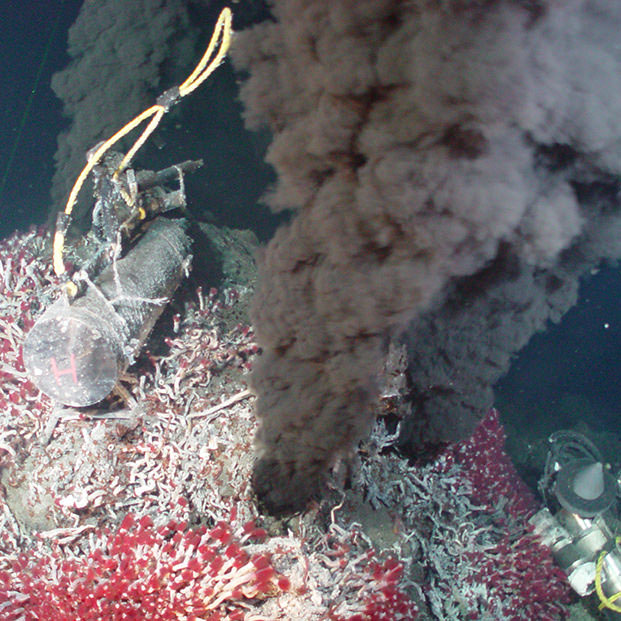
If the mantle is largely solid, something must be happening to cause melting at divergent boundaries. Adding water to hot rock can cause melting, but this does not not appear to be happening at divergent boundaries. Sea water does seep into the ocean crust at mid-ocean ridges. We know this because heated and particle-laden sea water rises back into the ocean at "black smoker" vents (see Figure 2.05 above and this video). But the sea water circulation hydrates mid-ocean ridge basalt, converting it to spilite, rather than causing the mantle to melt.
Because chemical equilibrium is approached by rocks at high temperatures, petrologists can use controlled experiments on rocks in the laboratory to determine
Figure 2.06. Effect of pressure on melting. The melting temperature of pure Mg-diopside is shown as a function of pressure. Notice that the melting temperature rises as pressure increases. Click on the diagram for a larger, interactive version with more information.