Figure 4.09 summarizes the equilibrium relations of the phases brine, halite, and ice that have been discussed in this chapter. It is, therefore, a phase diagram.
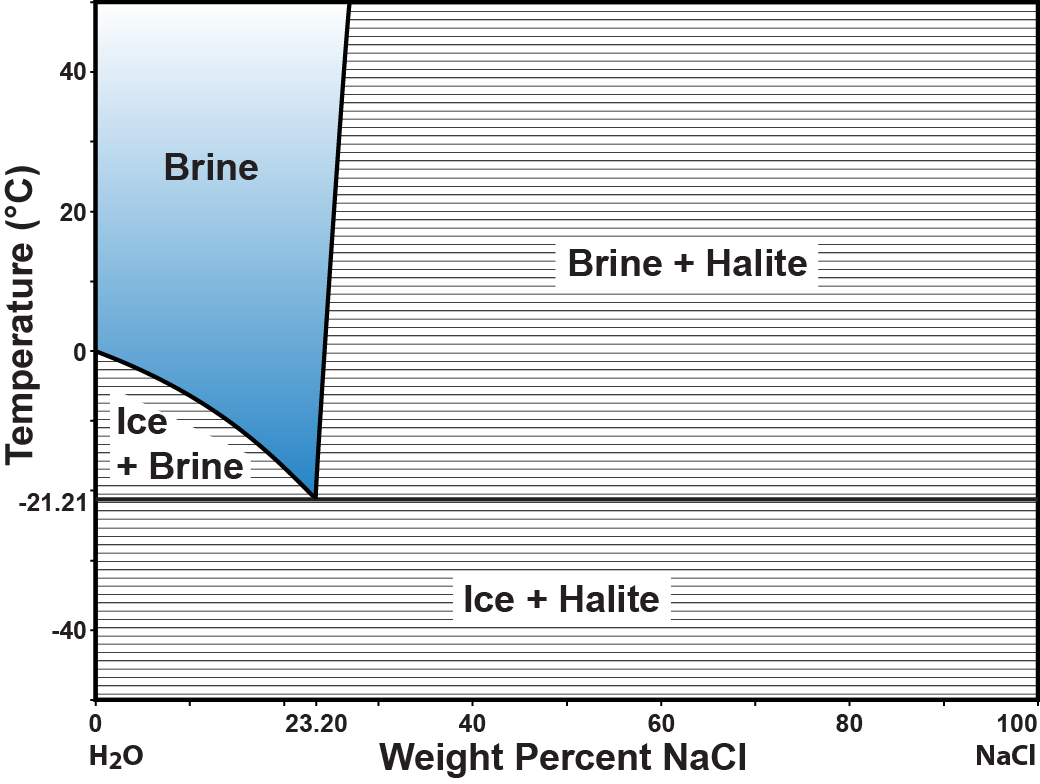
Figure 4.09. Caption needed.
What what phase or phases will coexist at equilibrium for a chemical composition of 10 weight percent NaCl at -10°C?
Yes, the point for 10 weight percent NaCl and -10°C plots in the 'Ice+Brine" field. No, the point for 10 weight percent NaCl and -10°C plots in the 'Ice+Brine" field. To see what phases will be present at equilibrium, plot the composition and temperature on the phase diagram and read the label on the diagram where the values plot.
Yes, the ice-saturated brine at -10°C would have a composition of 14 weight percent NaCl. No, the ice-saturated brine at -10°C would have a composition of 14 weight percent NaCl. You can find this value by using the "Coordinates" button in the enlarged version of a href="figure409.php">Figure 4.09. Look for the composition of the ice-saturation curve at -10°C.
Press "Enter" after you type in the number.
Good thinking. All mixtures of ice and halite will begin to melt at -21.21°C. The formation of liquid, in this case brine, is due to a reaction between the ice and the halite in the proportions 23.2 grams of halite to 76.8 grams of ice.
No. All mixtures of ice and halite will begin to melt at -21.21°C. The formation of liquid, in this case brine, is due to a reaction between the ice and the halite in the proportions 23.2 grams of halite to 76.8 grams of ice. Use the phase diagram. To determine the temperature of first melt, find the composition of the rock, then follow that composition up in temperature until it enters a field that contains liquid. In this case, all proportions of ice to halite (all compositions) yield the first liquid at -21.21C.