Chapter 4. Saturation Diagrams
By definition, igneous rocks have solidified from a magma, typically a molten (meaning hot) silicate liquid that may have crystals in it. Creating igneous rocks involves melting of rocks to make the magma, and crystallization of minerals to solidify the magma. This chapter examines melting and crystallization of familiar materials (e.g. water, ice, halite) to reveal important features of these processes and to develop graphical tools that are then used describe and think about melting of rocks and crystallization of magmas.
When most people are asked to tell what they know about melting and crystallizing, they will talk about ice and water. This makes sense because melting of ice and freezing of water are processes people see often, even in tropical climates because of freezers and ice cubes. There certainly are some similarities between melting and freezing of H2O and melting and freezing of rocks. In both cases, raising the temperature leads to melting and lowering the temperature leads to solidification. And in both cases, energy is consumed during melting and energy is released during solidification (melting is endothermic and solidification is exothermic). Of course, the temperatures are different. But it turns out that overall, for reasons other than temperature, pure H2O is not a good analog for thinking about the melting and crystallization of rocks.
There is a variable other than temperature that makes magma fundamentally different than water. You can see the difference in the following thin section views of solidified H2O and solidified basalt (Figures 4.01 and 4.02):
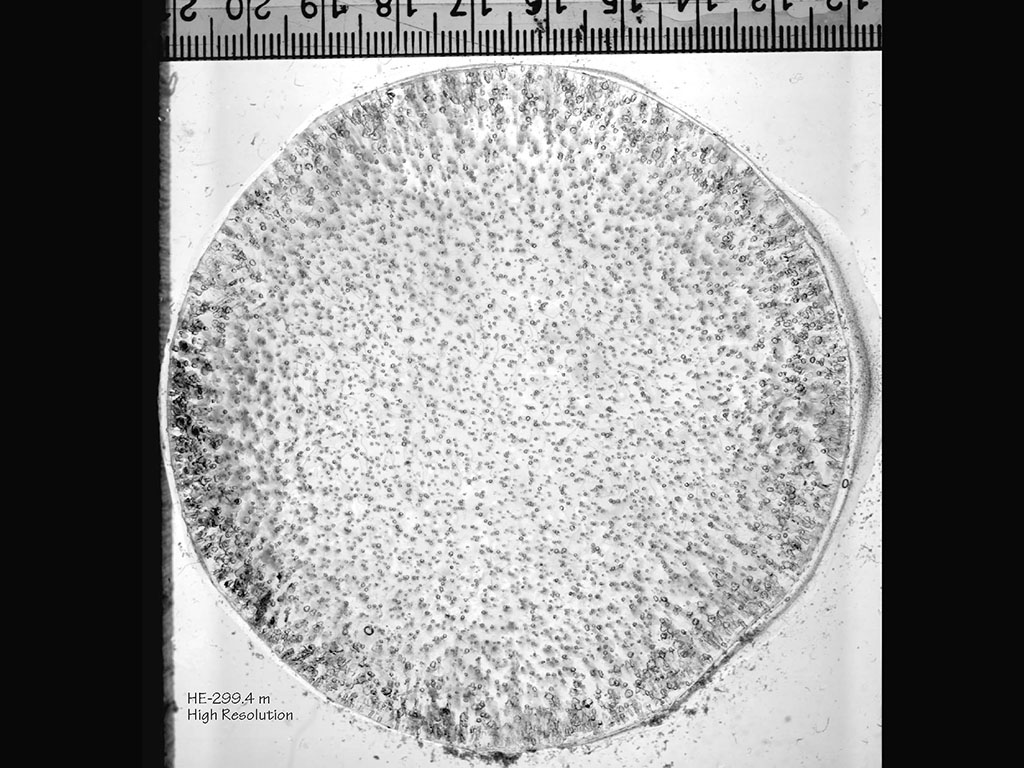
Figure 4.01. Glacial ice core thin section in plane polarized light (PPL). The core is approximately 9 cm across. Individual ice crystals are visible only in the crossed polarized light (XPL) view, which you can see by clicking on the image.
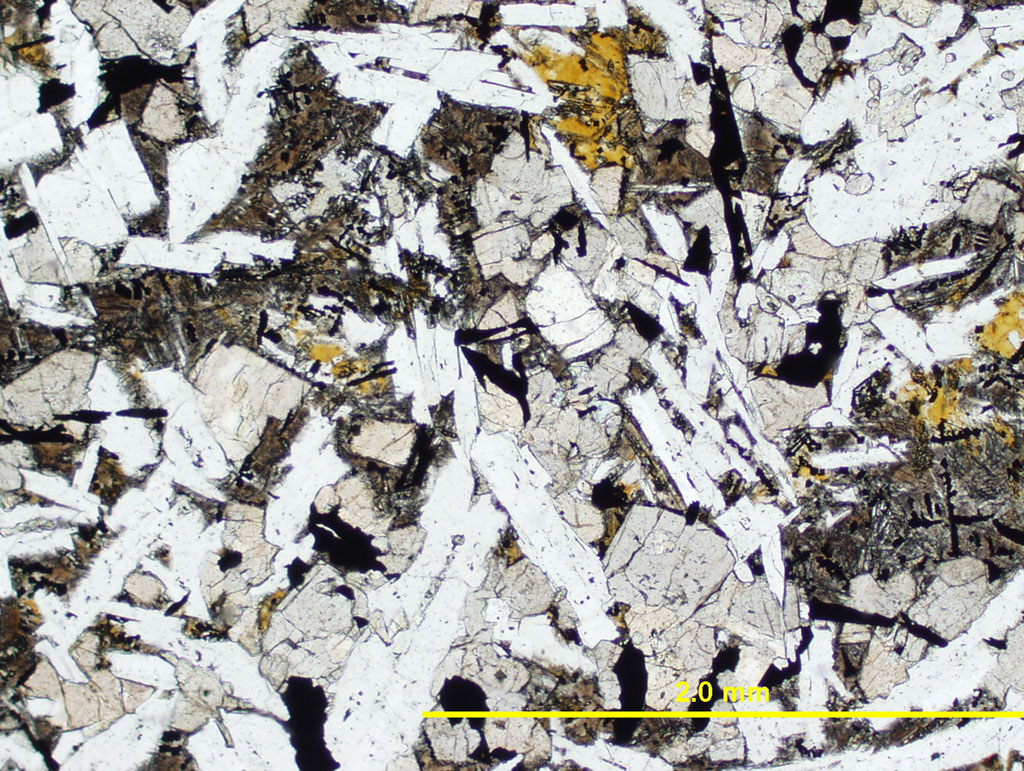
Figure 4.02. Basalt thin section in plane polarized light (PPL). Notice that it consists of several minerals: plagioclase laths (clear), clinopyroxene (beige), olivine (clear but equant), opaque minerals (magnetite and ilmenite), and "mesostasis" (partially devitrified glass). Click on the image to see a larger version with the option of a cross-polarized light (XPL) view.
The solified H2O on a frozen lake or in a glacier, such as the sample from Siple Dome in Antarctica in Figure 4.01, is a rock made of only one mineral (ice) with one chemical composition (H2O). The solidified magma (basalt) in Figure 4.02 is a rock made of several minerals (plagioclase, augite, olivine, magnetite), each with its own unique chemical composition. The fact that magmas solidify to several different minerals, each with a chemical composition different than the magma's compostion, makes magmas qualitatively different than water.
Let's examine a slightly more complicated case than pure H2O by adding salt (NaCl) to it. What would happen if you added the mineral halite (NaCl) to a glass filled with water and stirred the mixture? Please make a prediction and press "Enter":
Yes! Halite (table salt) will easily dissolve in the water.
The answer is dissolve. Halite (table salt) is denser (S.G. 2.16) than water (S.G. 1.00) and halite crystals will sink, not float, as they are dissolving in the water.
