Halite crystals are very soluble in water and will dissolve quickly if the glass of water is stirred. The water becomes "salty" (a brine) and some of its physical properties change. Properties that change include taste, density, refractive index, and electrical conductivity. The values of these properties depend on how much salt is added to the water. Mixtures of various proportions of halite and water (after stirring and settling) are shown in Figure 4.03.

Figure 4.03. Salt solutions (brines) made by mixing various proportions of halite (NaCl) and water. The masses in grams of NaCl and H2O for each solution are listed. Notice that undissolved halite crystals remain on the bottom of the beakers for some solutions.
The proportions of water and salt used for the mixtures in Figure 4.03 are indicated by the red circles on a graph of chemical composition vs. temperature in Figure 4.04. The horizontal axis is in units of weight percent NaCl. Weight percent NaCl is calculated with the formula:
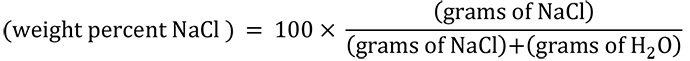
Figure 4.04. Chemical compositions of mixtures of water and halite (NaCl) in Figure 4.03 shown on a graph of weight percent NaCl vs. temperature. The solutions were all made at 21°C. The dashed line separates the mixtures with brines that are saturated with halite from the mixtures with brines that are not saturated with halite.
You can read the weight percent NaCl in a halite saturated solution at 21°C from Figure 4.04. Examine the enlarged version of Figure 4.04 and click on the Coordinates button to activate crosshairs when you move your mouse over the figure. Use the crosshairs to get an exact answer to the following question:
Press "Enter" after you type in the number.
Yes. Based on our experiments, a halite-saturated solution at 21°C will have 25 weight percent NaCl.
Press "Enter" after you type in the number.
No. You still have not given the correct answer. The dashed line in Figure 4.04 is located at 25 weight percent NaCl. This is the maximum amount of NaCl that can be dissolved in water at 21°C.