Because all the brines for the mixtures to the right of the dashed line are saturated with halite, all these brines have the same concentration of NaCl. That concentration is given by the dashed line. Because each of the mixtures to the right of the dashed line has a bulk composition with a different weight percent NaCl, the ratio of halite to brine must be different for each. This ratio can be determined graphically from the NaCl weight percent of the bulk composition relative to the NaCl weight percents of the halite and the brine. The relationsip between the proportions of halite and brine follows the "Lever Rule".
The Lever Rule is a simple graphical relationship between the proportions of phases in a mixture. If you have a good understanding of the Lever Rule, you will have a powerful tool at your disposal for the study of igneous and metamorphic rocks. Because the weight percent proportions must add up to 100, when there are only two phases there is only one variable.
Figure 4.05. Chemical compositions of mixtures of water and halite (NaCl) in Figure 4.03 shown on a graph of weight percent NaCl vs. temperature. The solutions were all made at 21°C. The dashed line separates the mixtures with brines that are saturated with halite from the mixtures with brines that are not saturated with halite. Click on the image to learn more about these relationships.
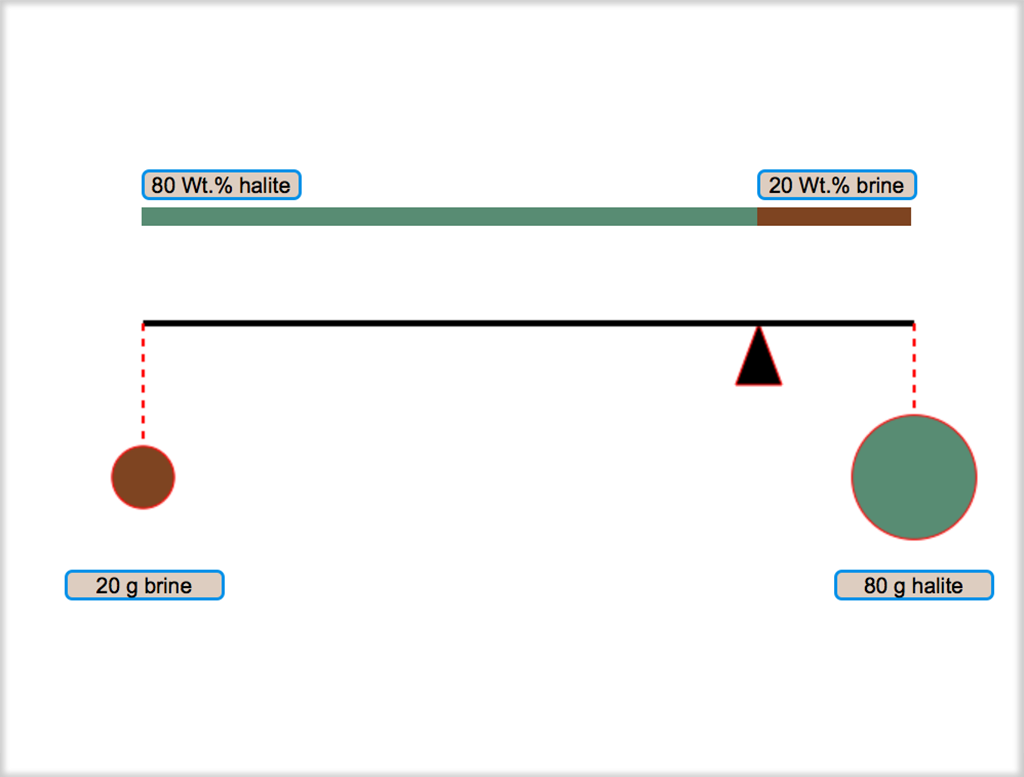
As you saw in the enlarged version of Figure 4.05, the bulk composition of a mixture of two phases divides the line between them (a tie line) into two parts whose relative lengths give the relative masses of the two phases. This relationship is called the Lever Rule because the mass of one phase would balance the mass of the other phase if the tie line were a lever and a fulcrum was placed at the position of the bulk composition. Figure 4.06 shows a lever and weights (circles whose areaa are proportional to the masses) for the brine-halite equilibrium shown in the previous figures. Click on Figure 4.06. Drag the fulcrum to change the masses and see how the Lever Rule works.
Press "Enter" after you type in the number.
Excellent! You show good understanding of the Lever Rule.
Press "Enter" after you type in the number.
Excellent! Now you are making sense of the the Lever Rule.
Use the graphical tools and try again.
Press "Enter" after you type in the number.
Excellent! Now you are making sense of the the Lever Rule.
- The brine has all the water (50g of H2O).
- The brine is saturated with halite and must, therefore, be 25 weight percent NaCl and 75 weight percent H2O.
- 75 weight percent of the brine is all the H2O (50g).
- The 25 weight percent of the brine that is NaCl must be 50g*(25/75) = 16.67g.
- The mass of the brine is 50g (H2O) + 16.67g (NaCl) = 66.67g
- The remaining NaCl (50g - 16.67g = 33.33g) must be in halite.
- The weight percent of brine in the mixture is 100*[66.67g/(66.67g + 33.33g)] = 66.67%.
You can check this result using the enlarged version of Figure 4.05. Click on the Coordinates button to locate the bulk composition (50 weight percent H2O and 50 weight percent NaCl. Click on the Lever Rule button. Drag the Lever Rule handle to match that bulk composition and you will be able to read the weight percent brine in the mixture.