Biological Sciences 330, Smith College
| Research in Cellular Neurophysiology
The Crayfish Swimmeret System.
REVISED: February 19, 2019
N1 motor neurons
backfilled with cobalt.
(Mulloney & Hall,
J Comp Neurol. 2000)
Click to enlarge.
 Cross
section of N1 showing bundles of motor and sensory
axons.
Cross
section of N1 showing bundles of motor and sensory
axons.
View the videos Crayfish Swimming and Swimmeret
Movements to see the rhythmic movements of the swimmerets.
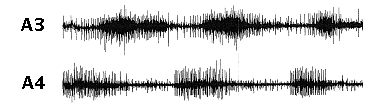
Listen to a
recording from the first roots (N1)
of the third (A3, top trace) and fourth (A4, bottom
trace) abdominal ganglia after activation of the
swimmeret motor pattern by 50 µM carbachol.
You will hear bursts of action potentials in the
axons of swimmeret motor neurons.
ANATOMY: The crayfish
swimmeret central pattern generator is an example of a
network of neurons that creates a behavioral pattern, the
rhythmic movement of the swimmerets. (In an isolated nerve
cord, the pattern is sometimes referred to as "fictive
swimming" since the muscles and appendages are no longer
attached.) Four abdominal ganglia (A2-A5) contain left and
right clusters of about 60 motor neurons each. Each
cluster is located near the base of the first root,
N1, in a region called the lateral neuropil (LN), as
shown in the micrograph at the left and the diagram below.
The motor neuron axons leave the ganglion in the first root,
which splits into an anterior branch that goes to the
swimmeret's return-stroke muscles (RS), and a
posterior branch to the power-stroke muscles (PS).
Recording from the two branches of N1 reveals alternating
bursts of spikes in the axons of the return-stroke and
power-stroke motor neurons. These bursts activate muscle
contractions, producing alternating forward and backward
movements of the swimmerets.
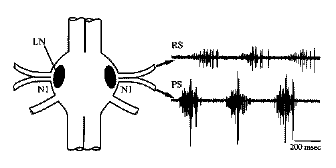
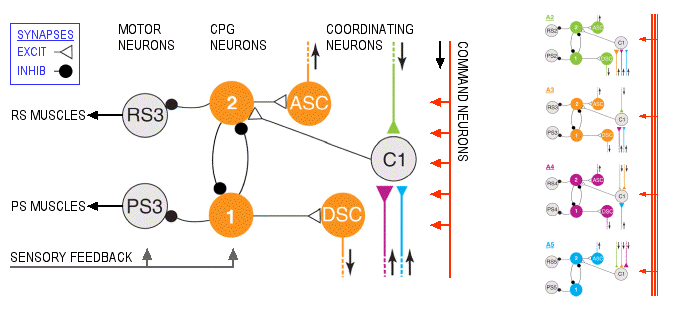
An abdominal ganglion, showing the lateral neuropil (LN) where the swimmeret motor neurons are located, the first roots (N1) carrying axons from the motor neurons, and examples of extracellular recordings from the return-stroke (RS) and power-stroke (PS) branches of the first root. Source: Mulloney B, Skinner FK, Namba H, Hall WM (1998) Intersegmental coordination of swimmeret movements: mathematical models and neural circuits. Ann N Y Acad Sci 860:266-80.
The excitatory motor neurons release glutamate at the muscle cells, causing them to depolarize and contract. There are about 30 excitatory motor neurons in each RS and each PS group. Like other crustacean muscles, the swimmeret muscles also receive axons from two or three large inhibitory motor neurons. These neurons release GABA at the muscle cells, opposing depolarization and reducing contraction. When the excitors to one class of muscles are active, often the inhibitors to the antagonist muscle are also active.
CENTRAL PATTERN
GENERATOR: The RS and PS motor neurons in each LN
cluster are driven by four non-spiking local
interneurons to form a pattern-generating module.
The modules on each side of a ganglion and in adjacent
ganglia are linked to each other by coordinating neurons. In
addition, five command interneurons run the length of the
nerve cord and send branches to activate the swimmeret
modules.
|
|
|
|
A pattern-generating
module. This example is
from the left side of the third ganglion (A3). |
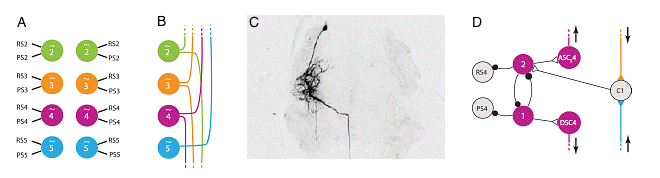
Four pattern-generating modules on the left side of the abdominal nerve cord, showing the coordinating neurons that establish a phase relationship between adjacent swimmerets, and the command neurons that activate swimming. |
|
Modified from: Smarandache, C, Hall, WM, and Mulloney, B (2009) Coordination of rhythmic motor activity by gradients of synaptic strength in a neural circuit that couples modular neural oscillators. J. Neuroscience 29: 9351-9360, Fig 9. See another diagram of the pattern generating module. (Adapted from Mulloney, B (2003) During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J Neuroscience 23(13): 5953-5962, Figure 10.) |
|
In each pattern generating module, the two pairs of non-spiking local interneurons (1 and 2) inhibit their respective clusters of motor neurons and also each other. The circuit is an example of reciprocal inhibition, which guarantees that only one pair of local interneurons (1 or 2) and thus one set of motor neurons (RS or PS) will be active at one time. This establishes the pattern of alternating bursts of RS and PS spikes, leading to alternating forward and backward swimmeret movements. Interestingly, it is not yet known which neurons excite the motor neurons, since only inhibitory connections to motor neurons have been discovered from the local interneurons.
The system lends itself to pharmacological intervention. Acetylcholine (and its analog, carbachol) turn on the swimming CPG, as does the peptide proctolin. Proctolin, a five-amino-acid peptide, is known to be contained in three of the five pairs of command neurons that activate the swimmeret system, so it is plausible that applying proctolin to a ganglion mimics the release of this transmitter from command neurons to activate the CPG system. It is also known that acetylcholine directly depolarizes RS and PS motor neurons by acting on muscarinic receptors. However, it is not yet clear what the source of that acetylcholine might be. At the bottom left of the figure, sensory feedback from stretch receptors and other sensory neurons is shown connecting (in an undefined way) to the motor neurons and local interneurons. It is likely that at least some of these sensory neurons release ACh as their transmitter, but this is unlikely to be the sole source of cholinergic excitation of the CPG circuits..
COORDINATION: The
local interneurons also excite coordinating
interneurons that fire bursts of spikes simultaneously
with the return stroke (DSC, descending to the next
posterior ganglion) or the power stroke (ASC,
ascending to the next anterior ganglion). In the target
ganglia, the ASC and DSC axons synapse on non-spiking
commisural interneurons (C1) that convey timing
information to the CPG module.
|
|
|
Coordinating interneurons. A Diagram emphasizing that four abdominal ganglia (A2-A5) each contain independent left and right modules for generating the swimmeret pattern. B Each module sends ascending and descending axons to adjacent ganglia. Only modules from the left side of the nerve cord are shown. C A filled example of a descending (DSC) interneuron, viewed from the dorsal surface of the ganglion. The neuron's cell body is at the top (anterior); its neurite branches extensively in the lateral neuropil (center left), and its axon leaves in the connective to the next ganglion (posterior, bottom). D The CPG module from the left side of ganglion 4, showing coordinating neurons (ASC4, DSC4) sending axons to adjacent ganglia, and a commissural interneuron (C1) receiving excitatory synapses from adjacent ganglia. Modified from: Smarandache, C, Hall, WM, and Mulloney, B (2009) Coordination of rhythmic motor activity by gradients of synaptic strength in a neural circuit that couples modular neural oscillators. J. Neuroscience 29: 9351-9360, Fig 2. |
In experiments, the motor pattern can be recorded from the first roots of abdominal ganglia A2-A5 using suction or pin electrodes. Adding the acetylcholine mimic carbachol or the peptide proctolin to the bath can activate the CPG pattern. It is not yet certain where those drugs act on the swimmeret system, although (as mentioned) some command neurons contain proctolin, and motor neurons (and probably some interneurons) respond to acetylcholine.
Link
© 2003 - 2016, 2019 by Richard F. Olivo. Permission is granted to non-profit educational institutions to reproduce or adapt this Web page for internal use provided that the original source and copyright are acknowledged.